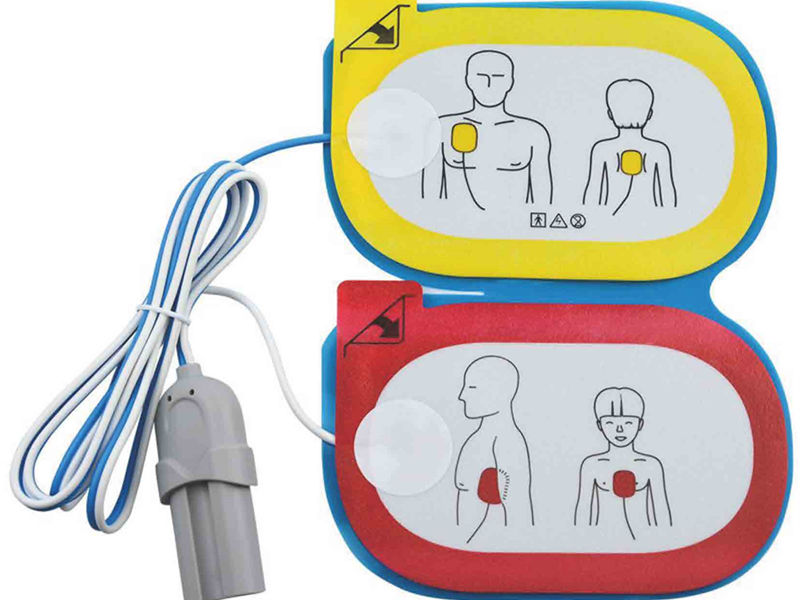
Recently, the disposable defibrillation electrode tablet independently developed and designed by MedLinket has successfully passed the registration of China National Drug Administration (NMPA).
Product Name: disposable defibrillation electrode
Main structure: it is composed of electrode sheet, lead wire and connector plug.
Scope of application: it can be used in external defibrillation, cardioversion and pacing.
Applicable population: patients weighing more than 25kg
The above is the illustration of MedLinket disposable defibrillation electrode tablets. If you want to know more matching models of defibrillation electrode tablets, you can contact your sales representative at any time or send an email to sales@med -Linket.com, we will provide you with professional services.
MedLinket has always insisted on providing customers with first-class products and services, and fulfilled the mission of “making medical care easier and people healthier”. Adhering to rigorous, efficient and professional services, we will work with you to promote safe, effective and compliant medical devices to the market at the fastest speed and contribute to the development of global human health.
Thank you for your support and trust!
Shenzhen Med-link Electronics Tech Co., Ltd
October 27, 2021
发布时间: 21-11-01